Method for preparing metal titanium
Titanium and its alloys having excellent corrosion resistance properties of low density, high temperature and the like. The world titanium industry is experiencing a single model with aerospace as its main market, and has transitioned to a multi-modal model focusing on the development of metallurgy, energy, transportation, chemicals, biomedicine and other civilian fields. At present, only a few countries in the world that can produce industrialized titanium are the United States, Japan, Russia, China and other countries. The total annual output of titanium is only tens of thousands of tons. However, due to the great strategic value of titanium and its position in the national economy, titanium will become the "third metal" after the rise of iron and aluminum , and the 21st century will be the century of titanium.
Current Titanium Production Process Titanium is currently produced by metal thermal reduction, which refers to the preparation of metal M by the reaction of a metal reducing agent (R) with a metal oxide or chloride (MX). The titanium metallurgical methods that have been industrially produced are the magnesium thermal reduction method (Kroll method) and the sodium thermal reduction method (Hunter method). Because the Hunter method is more expensive to produce than the Kroll method, the only method currently widely used in the industry is the Kroll method. Since its development in 1948, the Kroll method has been criticized for its high cost and low reduction efficiency. Half a century has passed, and there has been no fundamental change in the process. It is still intermittent production and has not been able to achieve continuous production.
New Trends in Metal Titanium Production Process The world's titanium industry has evolved over the past few decades. Although a series of improvements have been made to the Kroll and Hunter methods, they are intermittent operations, and small improvements do not significantly reduce the price of titanium. Therefore, a new, low-cost continuous process should be developed to fundamentally solve the problem of high production costs. To this end, the researchers conducted a large number of experiments and research. The current research focuses on the following methods: Electrochemical reduction method In order to reduce costs, people have studied the direct deoxidation of titanium metal. Electrochemical methods have been used abroad to reduce the concentration of dissolved oxygen in titanium below the detection limit (500 ppm). They believe that in the process of electrochemical oxygen scavenging, the oxygen scavenger calcium is produced when electrolytic calcium chloride is molten, and O 2- is precipitated as CO 2 or CO at the anode. This new high-purification method, not only for deoxidation of titanium, but also for yttrium, a rare earth metal neodymium, and oxygen content can be reduced to 10ppm.
The industrialization experiment of the electrochemical method is as follows: first, the titanium dioxide powder is cast or pressure-formed, sintered as a cathode, graphite as an anode, CaCl2 as a molten salt, and electrolysis in graphite or titanium crucible. The applied voltage is 2.8V to 3.2V, which is lower than the decomposition voltage of CaCl 2 (3.2V to 3.3V). After electrolysis for a certain period of time, the cathode was changed from white to gray, and observed under SEM, 0.25 μm of TiO 2 was converted into 12 μm of titanium sponge. The main reason for the use of calcium chloride as a molten salt is that its price is low, and it has a certain solubility to O 2- , so that the precipitated titanium is not easily oxidized; in addition, CaCl 2 is non-toxic and has no pollution to the environment.
Compared with TiCl 4 molten salt electrolysis, the raw material used in this method is oxide rather than volatile chloride, so the preparation process can be simplified, and the product quality is high; the redox reaction between the valence ions of titanium does not occur; The evolved gas is pure oxygen (inert anode) or a mixed gas of CO and CO 2 (graphite anode), which is easy to control and free from pollution.
This method not only promotes the reduction reaction in the vicinity of the cathode, but also deoxidizes the titanium obtained by the reduction. This method combines the direct electrolytic reduction of oxides with the electrochemical deoxidation method, and is a new method for preparing titanium, which has become the most attractive method in the titanium extraction process. According to the data published in the 2000 British Journal of Nature, using this method, the production cost per ton of sponge titanium is reduced by about 13,000 US dollars. At present, if the total output of 50,000 tons or 60,000 tons is produced by the electrochemical method, it will save 770 million annually. The cost of production of the dollar. [next]
The Armstrong method Amstrong et al. improved the Hunter method to make it a continuous production process. The process is: first, TiCl 4 gas is injected into the excess molten sodium, and the excess sodium serves to cool the reduced product and carry the product into the separation process. The product titanium powder is obtained by removing sodium and salt. The oxygen content of the product is at least 0.2%, which meets the standard of secondary titanium. A slight improvement in the process can produce VTi and AlTi alloys. Compared with the Hunter method, the method has the advantages of continuous production, low investment, wide application range of products, decomposition of by-products into sodium and chlorine gas for recycling.
The method is close to industrial production, but there are still several problems, such as how to further reduce the oxygen content, how the product costs and so on.
TiCl 4 electrolytic reduction method From the perspective of electrolysis process, the TiCl 4 electrolysis method is superior to the Kroll method and the Hunter method. Therefore, the development of the thermal reduction method from Kroll originally had the idea of ​​converting the smelting process of titanium into electrolysis.
The TiCl 4 electrolytic reduction method is the only method that was once considered to be a possible replacement for the Kroll process. The United States, the former Soviet Union, Japan, France, Italy, and China have conducted long-term and in-depth research. The use of TiCl 4 electrolytic reduction requires technically first to convert TiCl 4 to a lower chloride of titanium and dissolve it in the melt, while at the same time separating the cathode and anode regions and sealing the cell.
Some people in Italy have been working on the research of TiCl 4 electrolysis. They analyzed the electrolysis data of chlorination method and found that when the temperature is above 900 °C, there is no Ti 2+ or Ti 3+ in the electrolyte, only Ti 4+ and Ti. The electrolytic process established on the basis of this is that TiCl 4 gas is injected into the multilayer electrolyte and absorbed. This multi-phase layer is composed of potassium, calcium, titanium, chlorine, fluorine ions, potassium, calcium, etc., and separates the titanium cathode and the graphite anode. The liquid titanium formed at the lowest layer sinks into the bottom of the bath to a water-cooled copper crucible to form an ingot. However, the titanium obtained by this method is not high in purity and low in efficiency.
Prospecting for the superior performance and resource-rich titanium has attracted attention as an ideal material since the latter half of the 20th century, but so far it has not been rid of rare metals. The annual output of titanium in the world is only tens of thousands of tons. Because the Kroll method uses magnesium metal to reduce titanium tetrachloride to obtain sponge-like titanium metal, coupled with the superposition of process and many processes, the cost of titanium sponge is high, which affects the application of titanium in various industries. It has not been promoted in many application fields. However, we believe that with the development of science and technology, the development of new metal titanium production processes, the reduction of production costs, and the expansion of production scale, the 21st century will truly become the century of titanium.
Cold drawn steel bar is produced by hot rolled steel bar or wire rod to get smooth surface, more precision size, higher mechanical properties, which improves machining characteristics. It can also get various sections and sizes. So cold drawn steel bar is a better choice for machining users.
According to different steel grade, the production line is usually different. The Basic production line is as below:
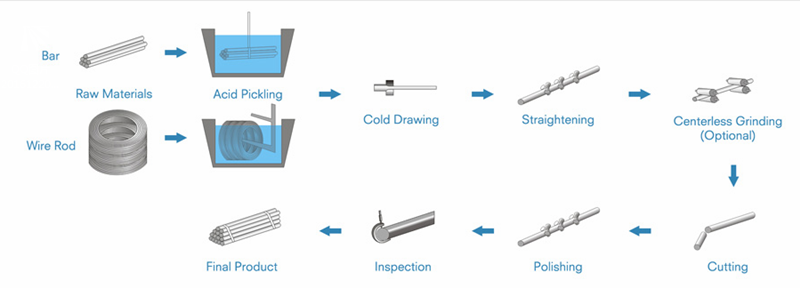
For high carbon steel or alloy steel bars, to avoid the cracks in inner or surface, the bars are usually required to be annealed before cold drawing.
To get different mechanical properties or hardness depending on final usage, the cold drawn bars will also be heat treated such as annealed, normalized or quenched and tempered(Q&T).
For CNC machines usage, to get much more precision sizes or much more better surface roughness for chrome plating, the cold drawn steel bars can also be centerless ground or polished.
We have more advantages on producing cold drawn steel bars:
1) Big stocks of hot rolled round bars or wire rods as raw materials
2) Wide range of cold drawn steel bar sizes: from 10mm to 150mm
3) Different cold drawing medias powder or oil to get different surface
4) Straightening machines to get better straightness up to 0.5mm/m
5) Grinding and polishing machines to get better roughness upto 0.4um
6) Heat treating furnaces to adjust the mechanical properties
7) Full sets of testing equipment to test the sizes, mechanical properties and microstructure.
8) Multiple packages to avoid broken packages and anti-rusty
Cold Drawn Bar,Round Steel Bar,Cold Drawn Steel Bar,Aisi 1018 Steel Square Bar
SHANDONG LE REN SPECIAL STEEL CO., LTD. , https://www.sdthreadedrods.com
